There’s been lots of talk of late about a hydrogen economy. I’ve looked into this. In a nutshell I can’t see Hydrogen being widely adopted for energy storage within our electricity system. Likewise, I can’t see it being widely used for transport except in some very limited cases. Its use within the gas network seems like greenwash. However there does seem to be some potential in using hydrogen as an intermediate product in creating transportable liquids like ammonia, methanol and methane for use as export feedstocks or as fuels.
by Richard Keech
Published 2018-09-23, based on investigation done in early 2017
The promise of hydrogen. Hydrogen gas (H2) has been widely proposed as a renewable fuel and industrial feedstock produced by using water and renewable electricity. Talk of the ‘hydrogen economy’ has been around for years based on the idea that H2 offers better prospects than the combination of renewable electricity and (chemical) batteries.
Electricity to hydrogen. The direct application of electricity via electrolysis of water yields hydrogen and oxygen plus heat. Where electricity is abundant then hydrogen can be used as an energy storage and transfer medium. The efficiency of electrolysis is reported to be in the range 40% to 67% (Bertuccioli, 2014). The most common electrolyser technology is alkaline electrolysis. Proton Exchange Membrane (PEM) electrolysis is more efficient. Other technologies are in the pipeline. There does not appear to be any in-production large-scale use of electrolysis for hydrogen as a fuel. Most (>90%) industrial hydrogen production is currently from steam reforming of methane (SRM) and is used as an industrial feedstock not as a fuel.
Hydrogen properties. Hydrogen gas has an energy density (by weight) about three times that of gasoline. Hydrogen molecules are very small so proper containment is more difficult than for other fuels. Hydrogen combusts in air across a very wide range of concentrations (4% to 75%). For comparison, gasoline is explosive only between 1.4% and 7.6% concentrations (H2tools). Its very low density means that leaking gas will rise quickly and dissipate if a pathway exists. Hydrogen burns in air with only a very faint blue flame which is almost invisible in daylight. Hydrogen flames emit in the UV band, and very little in the near-IR band making them much harder to avoid. The minimum energy required for hydrogen ignition (0.02mJ) is about one tenth that required for gasoline. Hydrogen isn’t corrosive but it can make some metals brittle. Hydrogen is non-toxic, but could represent an asphyxiation hazard.
| Fuel | Energy density [higher heating value MJ/kg] |
| Hydrogen | 142 |
| Methane | 55 |
| Propane | 50 |
| Gasoline | 47 |
Compression. The volumetric density of H2 is 0.090g/L at standard temperature and pressure (STP). Therefore volumetric energy density at STP is only 12.7J/L. The very low volumetric density of H2 at atmospheric pressure dictates that very high-ratio compression is necessary for practical storage and transportation. The commonly seen pressures used for hydrogen refuelling stations (HRSs) are 350bar and 700bar (SAE J2061). Compression to 700bar requires energy equivalent to about 16% of the energy in the hydrogen (Bossel and Eliasson 2003).
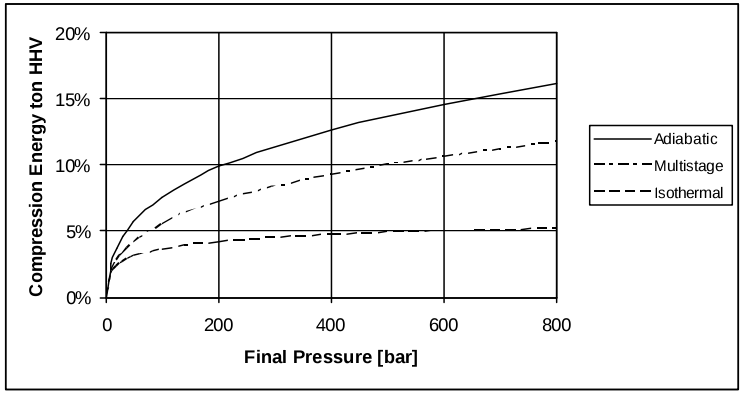
Figure 1: Energy loss from hydrogen compression (from Bossel and Eliason)
Fuel Cells. Hydrogen can serve as a means of direct electrical generation through the use of fuel cells. Fuel cells take hydrogen and air and generate electricity and water. Their efficiency is about 47% (Pellow 2015). Fuel cell technology is still maturing. The most widely used fuel cells are based on a technology that depends on non-trivial amounts of platinum as a catalyst. As a result they are currently very expensive. Creating low-cost fuel-cell technology is the subject of much active research.
Hydrogen-methane blending. A possible role for hydrogen is to blend with methane (‘natural gas’). This might be done to reduce the emission intensity of the gas and/or to increase the output of large renewable energy generators. This appears to be viable (Melaina 2013) at concentrations up to 15% without modifying the existing gas infrastructure or appliances. It has also been proposed that hydrogen distribution could take place through the gas network by blending, and then separating at the point of use. Leakage could be an issue because hydrogen permeation rates are 4 to 5 times that of methane (op cit) in typical polymer pipes.
Hydrogen for electrical energy storage. It is possible to use H2 for effective storage of (perhaps surplus) electrical energy. An electrolyser creates the H2, and a fuel cell turns it back into electricity. However the round-trip energy efficiency of this process is about 30%, compared to about 83% for lithium batteries (Pellow 2015).
Hydrogen as a transport fuel. There has been an enormous investment in the development of Hydrogen Fuel Cell (HFC) vehicle technology based around H2 as a fuel. The promise of HFCs is that they offer long driving range, rapid refuelling with a (potentially) emission-free fuel for fuel-cell vehicles (FCVs). Currently Honda, Toyota and Hyundai have FCVs (Clarity, Mirai and ix35 respectively) in very limited production. They are not available in Australia though Hyundai have announced the ix35 in Australia. In an FCV, H2 is stored in high-pressure tanks, typically made using filament-wound carbon fibre. The Hyundai ix35, for example, stores 5.6kg of H2 at 700bar. For comparison, LPG-fuelled vehicles store that fuel in tanks at about 6bar to 15bar depending on the temperature (Elgas).

Figure 2: Filament-wound tank from a Hyundai ix35 FCV (image credit: Hyundai)
Transportation of Hydrogen. The low volumetric density of hydrogen increases fuel transportation costs. It has been estimated (Bossel and Eliasson) that at 40-tonne delivery truck, capable of carrying 25t of gasoline, would only carry 300kg – 500kg of H2. Even allowing for H2’s high energy capacity (by weight), this represents a significant efficiency penalty. This might not be so much of an issue if H2 can be generated onsite.
Hydrogen to other fuels. Creation of H2 from renewable energy can be an intermediate step in the creation of other, more familiar fuels such as methane and methanol. This is discussed further in the next section.
Market Timing. If the automotive market reaches an EV tipping point in the near future, then it’s unlikely that FCVs, even if they become mature and affordable, will be able to prevent EVs from achieving market dominance. So it might not be sufficient for FCVs to be as good as EVs if they ‘miss the boat’.
Issues with Hydrogen
Efficiency. The use of hydrogen as a fuel presents some practical problems. For stationary energy-storage applications the very low round-trip efficiency (about 25% – 30%) is a grave limitation. In this application, hydrogen would need to compete against pumped hydro and batteries – both of which offer round-trip efficiencies greater than 80%. The same efficiency issues apply when hydrogen is used in FCVs for transport. So EVs are about three times more energy efficient than FCVs if electrolysis losses are included (Seba 2015).
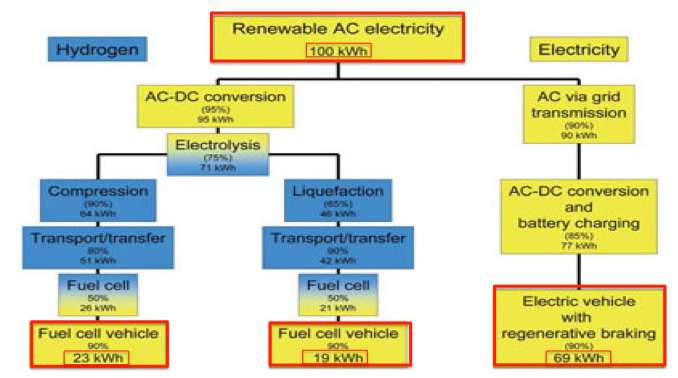
Figure 3: Comparative efficiency of FCVs and EVs (image credit: Tony Seba 2016)
Infrastructure. Hydrogen fuel suffers from the ‘chicken and egg’ problem. Without a widespread network of affordable fuelling stations, there will be no demand for FCVs. And without the FCVs there’s no commercial incentive to build the refuelling infrastructure. Whereas EVs can charge at home with zero or very small infrastructure investment, there is no reasonable prospect of FCVs filling at home. Creation of a global hydrogen refuelling infrastructure, comparable to today’s gasoline distribution would be extremely expensive.
Shrinking business case. The core case for hydrogen as a transport fuel centres on it having long range and quick refuel compared with EVs. Rapid advances in EV range and refuelling appear to diminish the business opportunity for FCVs. For example the Tesla Model 3, due in Australia in 2019, is expected to have a range of about 350km, and charging at a rate of about 500km of range per hour of charge (using a Supercharger). The capability of overnight, at-home EV charging also tends to negate the quick refuel benefit of an FCV.
Cost. It’s not clear what the long-term at-the-pump cost of H2 will be, nor what the cost of FCVs will be. However there seem to be no likelihood that FCVs could possibly have a significant life-cycle cost advantage over EVs. A 2016 report described a cost of USD16.63/kg at the pump (Wong) though this doesn’t represent a realistic market price.
Safety. The physical properties of hydrogen gas present many challenges to make systems safe. Foremost seems to be the relative difficulty in avoiding leaks, combined with its highly flammable nature. With appropriate engineered measures the use of hydrogen can, no doubt, be sufficiently safe. However these safety systems will be costly. In automotive applications, hydrogen may, in one way, be safer than gasoline because H2 will very quickly dissipate if a tank is ruptured.
Non-fossil methane and methanol
Pathways. An alternative to hydrogen and biofuels is the direct use of renewable energy in the synthesis of fuels such as methane and methanol. A number of possible pathways exist which could offer a direct pathway to renewable fuels. One such pathway is synthesis of methane from renewably sourced hydrogen. Another possible pathway involves methanol synthesis from hydrogen. Other pathways may exist.
Methane synthesis
Sabatier reaction. There is a chemically well-understood reaction, the Sabatier reaction, which takes H2 and CO2 and gives methane and water and heat. Given a source of renewable H2 (discussed in the previous section) it seems an attractive proposition to produce renewable methane and water, and in the process consume CO2.
Synthetic methane. As discussed in the previous section, there is some merit to mixing hydrogen with methane to lower the effective emissions of methane. However this has only marginal utility compared with complete substitution of existing fossil methane with renewable methane. The carbon emitted when burning methane is fully offset by the carbon used in the creation of the methane in the first place, so the methane becomes carbon-emission neutral (so long as there are negligible leaks).
Infrastructure utilisation. Methane, then, has multiple possible sources – fossil, bio, hydrogen-synthesised. Given the widespread dependence upon methane and the extensive latent infrastructure and appliances built around methane, it would seem to be extremely attractive to be able to directly substitute fossil methane with synthetic- or bio-methane to the extent that they are available. This avoids great cost by utilising existing infrastructure, compared with creating a new energy infrastructure.
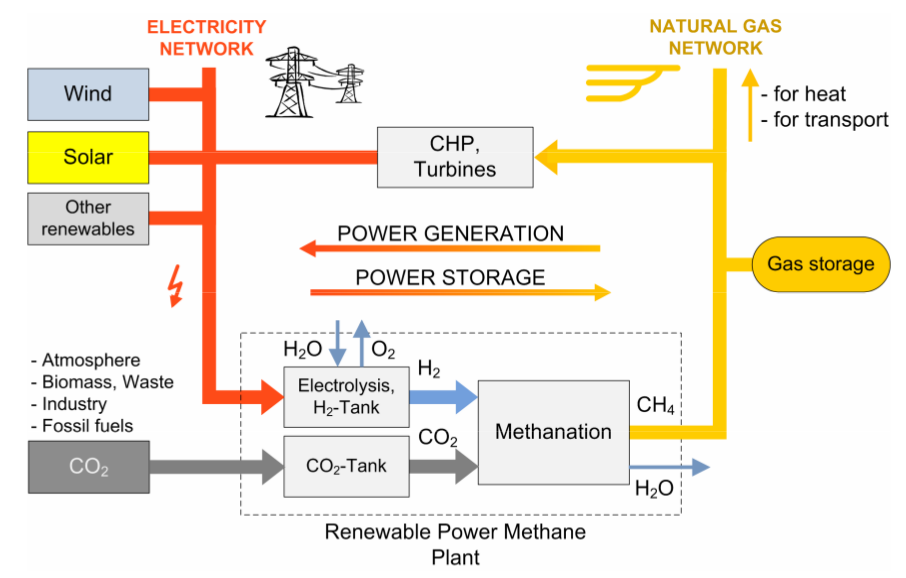
Figure 4: Renewable production of methane (image credit: Sterner 2009)
Methanol synthesis
A similar approach to methane synthesis can be applied to produce methanol instead. Whereas methane is very useful for stationary energy use, it is of much less use for transport. This is where methanol can be useful – either directly as a fuel blend, or as a feedstock for production of biodiesel.
Precedent: Vulcanol. The firm CRI in Iceland produces renewable methanol known as Vulcanol. Their Georg Olah plant has been producing methanol since 2012. It has a current capacity of 5ML/annum, making it the largest plant of its type. The power used in the production is all from hydro and geothermal plants (CRI 2017). There is a small net carbon emission in the process because the geothermal resource emits CO2. However the net emissions from methanol production are 90% less than if the input energy was fossil-derived.

Figure 5: The Georg Olah methanol (‘Vulcanol’) plant in Iceland (image credit: CRI)
References
Seba, A., 2015 ‘Toyota vs. Tesla – Can Hydrogen Fuel-Cell Vehicles Compete with Electric Vehicles?’, http://tonyseba.com/toyota-vs-tesla-can-hydrogen-fuel-cell-vehicles-compete-with-electric-vehicles/
April 25, 2017 , “Scientist invents way to trigger artificial photosynthesis to clean air”, https://phys.org/news/2017-04-scientist-trigger-artificial-photosynthesis-air.html
Dunis, V., 2017, “Could South Australia be the nation’s hydrogen state, too?”, Renew Economy, http://reneweconomy.com.au/south-australia-nations-hydrogen-state-11243
Sterner, M., 2009, “Bioenergy and renewable power methane in integrated100% renewable energy systems: Limiting global warming by transforming energy systems”, University of Kassel, Faculty of Engineering and Computer Science, http://www.uni-kassel.de/upress/online/frei/978-3-89958-798-2.volltext.frei.pdf
Elgas, ‘How Much Pressure is in LPG-Propane Cylinders?’, http://www.elgas.com.au/blog/1969-how-much-pressure-is-in-lpg-propane-cylinders-in-what-state
CRI, 2017, http://carbonrecycling.is/
Bossel, U. and Eliason, B., 2003, ‘Energy and the Hydrogen Economy’, http://www.afdc.energy.gov/pdfs/hyd_economy_bossel_eliasson.pdf
Pfieffer, D., 2006, ‘The Myth of the Hydrogen Economy’, Resience, http://www.resilience.org/stories/2006-01-03/myth-hydrogen-economy/
Pellow, M., et al., 2015, ‘Hydrogen or batteries for grid storage? A net energy analysis”, DOI: 10.1039/C4EE04041D (Analysis) Energy Environ. Sci., 2015, 8, 1938-1952, http://pubs.rsc.org/en/content/articlehtml/2015/ee/c4ee04041d
Phys.org, 2006, ‘Why a hydrogen economy doesn’t make sense’, https://phys.org/news/2006-12-hydrogen-economy-doesnt.html
Wong, ‘Fill ‘er Up: Refueling the 2016 Toyota Mirai’, https://www.cars.com/articles/fill-er-up-refueling-the-2016-toyota-mirai-1420690448036/
H2Tools, ‘Hydrogen compared to other fuels’, https://h2tools.org/bestpractices/h2properties
Bertuccioli, L., et al., 2014, “Development of water electrolysis in the European Union” . Client Fuel Cells and Hydrogen Joint Undertaking, http://www.fch-ju.eu/
sites/default/files/study%20electrolyser_0-Logos_0_0.pdf
Matthew Wade Logan et al., 2017, ‘Systematic Variation of the Optical Bandgap in Titanium Based Isoreticular Metal-Organic Frameworks for Photocatalytic Reduction of CO2 under Blue Light’, J. Mater. Chem. A (2017). DOI: 10.1039/C7TA00437K ; https://phys.org/news/2017-04-scientist-trigger-artificial-photosynthesis-air.html
Melaina, M., et al, 2013, ‘Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues’, NREL, http://www.nrel.gov/docs/fy13osti/51995.pdf
Office of the Chief Economist (OCE), 2016, ‘Australian Energy Statistics 2016’, Australian Government, https://industry.gov.au/Office-of-the-Chief-Economist/Publications/Documents/aes/2016-australian-energy-statistics.pdf
CEC, 2008, ‘Australian bioenergy roadmap : setting the direction for biomass in stationary energy to 2020 and beyond’, Clean Energy Council, http://trove.nla.gov.au/version/45678414
McReay, A., 2008, ‘Renewable Energy: A user’s guide’, Crowood Press, ISBN 9781847970619 (Chapter 4 Biofuels)
Thran, D. (ed)., 2015, ‘Smart Bioenergy: Technologies and concepts for a more flexible bioenergy provision in future energy systems’, Springer, ISBN 9783319161921
Government of Victoria, 2010, ‘Bioenergy from agriculture: the value chain’, Department of Agriculture, http://agriculture.vic.gov.au/agriculture/food-and-fibre-industries/industry-profiles/bioenergy-from-agriculture
Livingston, W.(ed), 2016, ‘The status of large scale biomass firing: The milling and combustion of biomass materials in large pulverised coal boilers’, IEA, ISBN 978-1-910154-26-7, http://www.ieabcc.nl/publications/IEA_Bioenergy_T32_cofiring_2016.pdf
Stuckly, C., et al., November 2012, ‘Bioenergy in Australia: Status and Opportunities’, ISBN 9780987435507, http://www.bioenergyaustralia.org/data/reports/BIOENERGY%20IN%20AUSTRALIA%20Rev%201.pdf